When your doctor prescribes a medication and the pharmacy says it’s out of stock, you don’t just want to hear "it’s on backorder." You need to know: Is this a local issue or a nationwide shortage? And if it’s nationwide, how long will it last? The FDA Drug Shortage Database is the only official federal source that answers these questions in real time.
What the FDA Drug Shortage Database Actually Shows
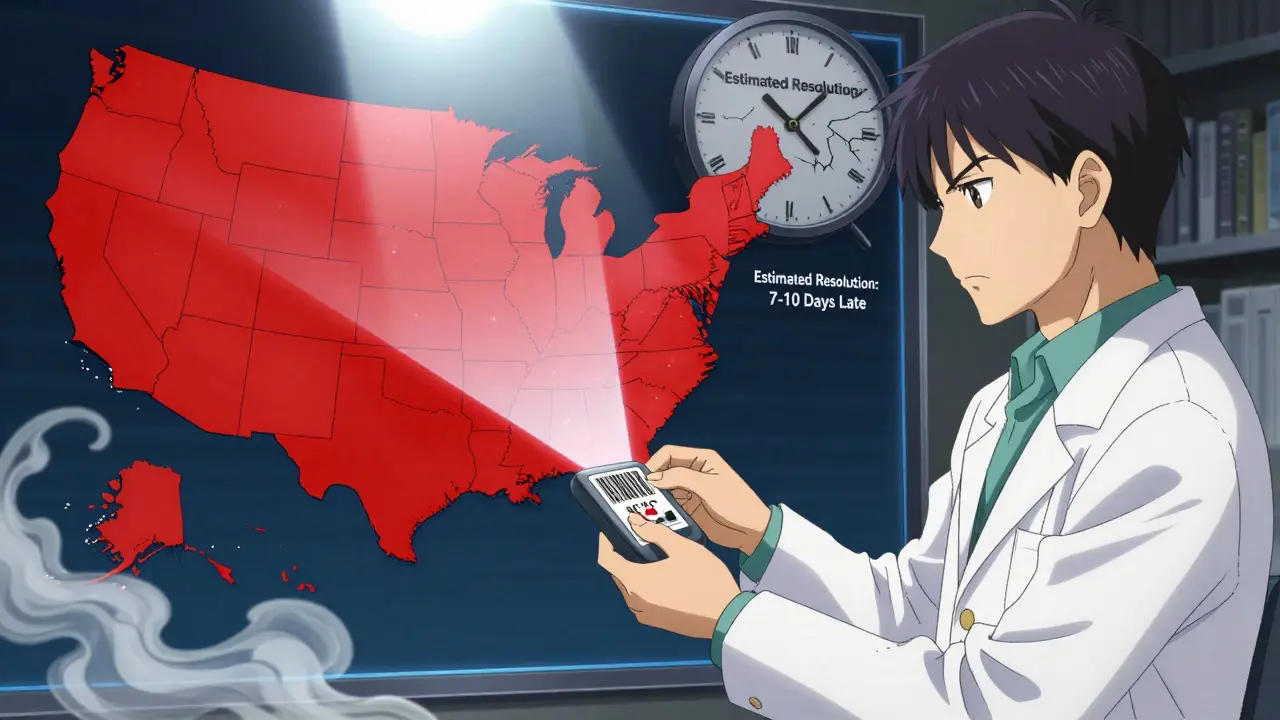
The FDA’s database isn’t a list of "sold out" items like you’d see on Amazon. It tracks drugs where demand is greater than supply across the entire U.S. - not just one store or region. As of early 2026, there are around 300 active shortages on the list, mostly generic injectables like insulin, antibiotics, and IV fluids. These aren’t rare drugs - they’re the ones hospitals and clinics rely on every day.
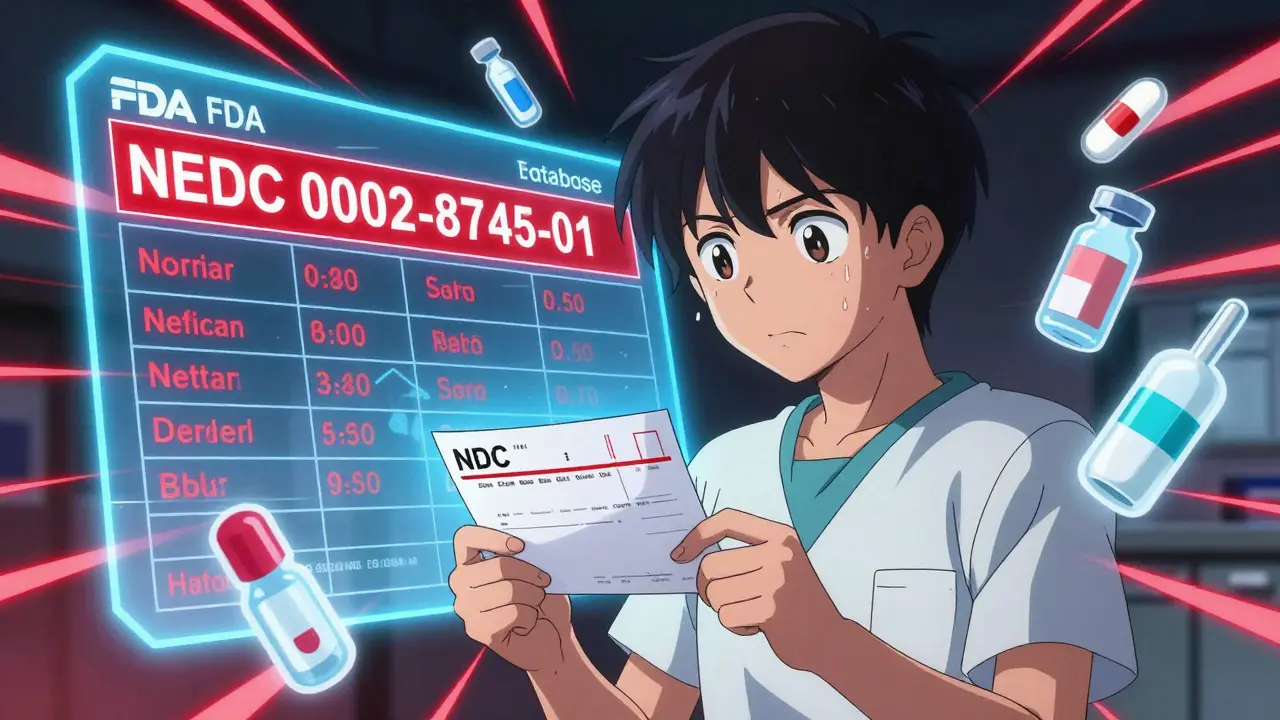
Each entry includes the drug’s generic name, the manufacturer’s name, and the exact National Drug Code (NDC) number. That last part matters. Two different brands of the same drug can have different NDCs - and only one might be short. For example, if you’re prescribed metformin 500mg tablets with NDC 0002-8745-01, you need to check that exact code. Just searching for "metformin" won’t tell you if your specific pill is available.
The database also tells you why the shortage exists. About two-thirds of all shortages are due to manufacturing or quality problems - things like contamination, equipment failure, or raw material delays. Only 8% are due to increased demand. The FDA updates this data daily, and manufacturers are legally required to report shortages under the FDASIA law. If they don’t, they can be fined up to $10,000 per day.
How to Access the Database
You don’t need special access. The FDA’s Drug Shortage Database is free and open to everyone. Here’s how to use it:
- Go to www.accessdata.fda.gov/scripts/drugshortages/default.cfm - this is the main website.
- Use the search bar to type in the generic name of the drug (e.g., "vancomycin") or the active ingredient.
- Filter results by therapeutic category (like "Cardiovascular" or "Infectious Disease") if you’re browsing broadly.
- Look for the NDC number on your prescription label and match it to the one listed.
- Check the status: "Current," "Resolved," or "Discontinued." "Resolved" doesn’t mean full supply is back - it just means supply meets minimum demand.
There’s also a free mobile app called "FDA Drug Shortages," available on iOS and Android. It’s especially useful for pharmacists and nurses on the go. You can turn on push notifications for specific drugs, scan NDC barcodes with your phone’s camera, and even report a shortage you’ve noticed that’s not yet listed.
What the Database Doesn’t Tell You
Here’s the catch: the FDA database is authoritative, but it’s not always timely. On average, there’s a 7-10 day lag between when a shortage starts and when it appears in the database. Why? Because manufacturers must confirm the issue, submit paperwork, and the FDA must verify it before publishing. By then, hospitals and pharmacies may already be scrambling.
Another limitation: the database doesn’t tell you what to use instead. If your insulin is short, it won’t suggest alternatives like Lantus or Humalog. That’s where other resources come in. The American Society of Health-System Pharmacists (ASHP) has a more user-friendly site with clinical guidance, substitution options, and dosage adjustments. Many providers check the FDA database first to confirm the shortage, then go to ASHP to figure out what to prescribe next.
Also, the FDA doesn’t track compounded drugs - those made in specialty pharmacies - or drugs that are only short in certain states. If you’re in rural Montana and your local pharmacy can’t get amoxicillin, but the FDA says it’s not short, that doesn’t mean the drug is available elsewhere. The FDA only lists nationwide shortages.
How to Use This Info in Real Life
If you’re a patient:
- Don’t panic if your pharmacy says a drug is out of stock. Check the FDA database yourself.
- If it’s listed as a shortage, ask your doctor about alternatives - and bring the NDC number with you.
- Don’t assume "resolved" means the drug is back to normal. Ask your pharmacy if they have enough to last your next refill.
If you’re a healthcare provider:
- Check the database before prescribing - especially for high-risk drugs like epinephrine or heparin.
- Use the "Reason for Shortage" field to avoid switching to another product from the same manufacturer. If one product from Company X is short due to contamination, others from the same company might be next.
- Subscribe to the FDA’s free email updates, sent every Tuesday and Friday. They list new shortages and resolved ones.
Pro tip: If you see a shortage that’s not listed, report it. Go to the FDA’s website and click "Report a Shortage." You’ll need your email and details about the drug, manufacturer, and where you’re seeing the shortage. The FDA reviews all reports and adds them if verified.

Why This Matters Right Now
Drug shortages have tripled since 2010. In 2025, over 1,200 new shortages were reported to the FDA - up from 800 in 2020. The biggest culprits? Manufacturing issues in India and China, supply chain delays, and consolidation among generic drug makers. The FDA’s database is the only tool that gives you a clear, legal picture of what’s really happening.
It’s not perfect. The estimated resolution dates are wrong nearly half the time. Predictive tools are still in testing. But it’s the best we have. And with new features launching in 2026 - like AI-driven alerts and barcode scanning in the app - it’s getting better fast.
For now, the key is knowing how to use it. Don’t wait for your pharmacy to call you. Don’t assume your doctor knows the latest status. Take five minutes to check the database yourself. It could save you time, money, and even your health.
Common Mistakes and How to Avoid Them
Even experienced pharmacists make these errors:
- Mistake: Thinking "resolved" means full supply is back. Fix: Call your supplier. "Resolved" just means supply meets minimum needs - not that it’s abundant.
- Mistake: Searching by brand name. Fix: Always use the generic name. The FDA database doesn’t list brand names.
- Mistake: Ignoring NDC numbers. Fix: Every dosage form (tablet, injection, concentration) has its own NDC. Match it exactly.
- Mistake: Not checking for extended use dates. Fix: Some drugs have FDA-approved extended expiration dates during shortages. Search "Extended Use Dates" on the FDA site.
For patients: If you’re on a long-term medication like warfarin or levothyroxine, keep a printed list of your NDC numbers. That way, you can quickly check availability during a shortage.
Is the FDA Drug Shortage Database free to use?
Yes. The FDA Drug Shortage Database is completely free for patients, pharmacists, doctors, and the public. No registration is required to search or view current shortages. The mobile app is also free and available on both iOS and Android.
How often is the FDA Drug Shortage Database updated?
The database is updated daily. New shortages are added as soon as the FDA verifies manufacturer reports, and resolved shortages are removed once supply meets demand. The mobile app syncs automatically, so you’ll see updates in real time.
Why isn’t my drug listed even though my pharmacy says it’s short?
The FDA only lists shortages that affect the entire U.S. market. If your pharmacy’s shortage is local - due to a delivery delay or low inventory - it won’t appear in the database. Also, there’s a 7-10 day reporting lag. Your drug may be short now but not yet officially reported. You can report it yourself using the "Report a Shortage" form on the FDA website.
Can I trust the estimated resolution dates?
The FDA’s estimated resolution dates are only about 79% accurate - and only for shortages reported directly through the manufacturer’s secure portal. For others, the estimate is often wrong. Don’t plan your treatment around these dates. Always confirm availability with your pharmacy closer to your refill date.
What’s the difference between the FDA and ASHP drug shortage lists?
The FDA lists only nationwide shortages that meet strict supply-demand thresholds. ASHP includes regional or temporary shortages, making its list larger - about 15-20% more drugs. ASHP also provides clinical advice on alternatives, while the FDA gives manufacturer and NDC details. Most providers use both: FDA to confirm a shortage, ASHP to find a replacement.
Do I need to register to use the FDA Drug Shortages app?
No. You can search, view shortages, and get notifications without registering. But if you want to report a shortage or save favorite drugs, you’ll need to verify your email address. The app works on iOS 12.0+ and Android 8.0+.
What should I do if I can’t find my drug on the FDA database?
If your drug isn’t listed, it may not be a nationwide shortage. Talk to your pharmacy - the issue could be local. If you believe it should be listed, report it using the FDA’s online form. Include the drug name, NDC, manufacturer, and where you’re experiencing the shortage. The FDA reviews all reports within 48 hours.






Just checked the FDA database for my mom’s insulin-listed as ‘current shortage.’ Called our pharmacy and they had it in stock, but warned it might be gone by Friday. Good to know the system’s not perfect, but at least you’re not totally in the dark.
Thanks for the heads-up on the NDC thing-I always just googled the drug name and assumed it was enough.
For anyone using this: if you’re on levothyroxine, keep your NDC on your phone. I had a shortage last year and the pharmacy switched me to a different brand without telling me. My TSH went haywire. Turns out the new one had a different filler that messed with absorption. FDA doesn’t warn you about that-your doctor should, but they don’t always know either. Save yourself the panic.
Really appreciate this breakdown. I work in a rural clinic and we get calls every week about ‘missing’ meds that aren’t actually shortages-just delivery delays. Sharing this with my team so we can stop wasting time chasing ghosts.
Also, the ASHP tip is gold. We’ve been using it for months now. Much better for clinical decision-making.
Five minutes to check the database could save your life. Seriously. I used to think it was just for pharmacists. Now I check before every refill. It’s not rocket science. Just don’t wait until you’re out.
Let’s be real-this is a symptom of late-stage capitalist pharmaceutical decay. The FDA’s database isn’t a solution; it’s a bandage on a hemorrhage. We’ve outsourced manufacturing to nations with lax oversight, consolidated supply chains into a handful of corporate actors, and now we’re told to ‘check the database’ like it’s some kind of civic duty. The system is broken. The database just makes you feel like you’re doing something while the machine keeps grinding.
And don’t get me started on the ‘extended expiration dates’-that’s just regulatory capture disguised as public safety. We’re being told to trust expired drugs because the supply chain can’t keep up. This isn’t innovation. It’s collapse with a UI.
Correction: the FDA database doesn’t update daily. It updates when manufacturers submit reports, which often happens weekly or biweekly. The ‘daily’ claim is misleading. Also, the 7–10 day lag isn’t ‘average’-it’s the norm. Most shortages are reported by patients or pharmacists, not manufacturers. The FDA doesn’t verify until after the fact. Don’t treat this as real-time data. It’s retrospective with a pretty interface.
Just wanted to say: if you’re a patient and you’re stressed about a shortage, you’re not alone. I’ve been there. But you’ve got tools now. Use the app, check the NDC, talk to your pharmacist. You’re not powerless. And if you report a shortage, you’re helping others too. Small actions matter.
OMG I just realized I’ve been searching by brand name this whole time. Like, I’ve been Googling ‘Lantus’ and wondering why nothing shows up. I’m 34 and this is the first time I’ve understood what an NDC even is. I feel like I’ve been living in a drug shortage simulation game and just got the tutorial.
So I reported a shortage of ampicillin last month because my clinic couldn’t get it. Got an email from the FDA 10 days later saying they verified it and added it to the list. Then two weeks later, it was gone from the list because some plant in India fixed their filter. That’s the whole cycle right there. The system works… eventually. And yeah, the resolution dates are usually wrong. But at least now I know why my doctor keeps changing my script.
It’s weird how something so technical-NDC codes, manufacturer reports, FDA verification-ends up being the difference between getting your meds and going without. We treat health like a commodity, but when it comes down to it, it’s just one person trying to get a pill that keeps them alive. The database doesn’t fix the system, but it gives you a little power back. And that’s worth something.
Wait… so the FDA doesn’t track compounded drugs? And they only list nationwide shortages? That means they’re ignoring rural areas where 70% of pharmacies can’t get basic antibiotics! This is a cover-up! They know this is happening, but they don’t want you to panic! They’re letting people die quietly so they don’t have to admit the system is collapsing! And the app? It’s probably collecting your data to sell to Big Pharma! I reported my shortage-now I’m getting spam emails from ‘PharmaCare Solutions’! This is surveillance capitalism disguised as public health!
Hey, if you’re reading this and you’re worried about your meds-breathe. You’re not alone. I’ve been on insulin for 15 years. I’ve had shortages. I’ve had to switch brands. I’ve cried in pharmacy parking lots. But here’s the thing: you’re smarter than the system. You’re checking this. You’re asking questions. You’re not just accepting ‘we’re out.’ That’s power. Keep going. You’ve got this. And if you need someone to talk to about it-I’m here.
So let me get this straight-you’re telling me that if my pharmacy says they’re out of metformin, I should just trust the FDA website? What if they’re lying? What if the manufacturer is hiding it? What if this is all part of a bigger plot to push people toward more expensive branded drugs? I’ve seen what happens when people rely on ‘official’ sources. Remember the opioid crisis? They said it was safe too. This feels… too convenient.
THIS IS WHY WE NEED TO BAN DRUG MANUFACTURING IN CHINA AND INDIA. 🇺🇸🇺🇸🇺🇸
WE USED TO MAKE OUR OWN MEDS. NOW WE’RE AT THE MERCY OF FOREIGN FACTORIES THAT CAN’T EVEN KEEP THEIR PLANTS CLEAN.
THE FDA ISN’T FIXING THIS-THEY’RE JUST DOCUMENTING OUR COLLAPSE.
STOP BUYING IMPORTED DRUGS. BUY AMERICAN.
🇺🇸 #MAKEAMERICAMEDSAGAIN
It’s worth noting that the FDA’s definition of ‘resolved’ is statistically meaningless. A drug is marked ‘resolved’ when supply meets the minimum threshold required by the CDC’s emergency allocation guidelines-not when it’s back to pre-shortage levels. In practical terms, that means pharmacies still have 2–3 days of stock left, which is enough to pass inspection but not enough to serve a normal patient load. The database is technically accurate but functionally deceptive. You’re being given a metric that looks like a solution but is really just a compliance checkbox.