ADPKD Treatment Suitability Checker
This tool helps determine whether Tolvaptan (Natrise) might be a suitable treatment option based on key health factors. Please answer the following questions:

TL;DR
- Tolvaptan (Natrise) is the only FDA‑approved drug that directly slows cyst growth in ADPKD.
- Key alternatives focus on supportive care, blood‑pressure control, or experimental vasopressin antagonists.
- When choosing, weigh efficacy, side‑effects, cost, and monitoring burden.
- Patients with early‑stage disease and good liver function benefit most from Tolvaptan.
- Always discuss with a nephrologist to tailor therapy to your disease stage and tolerance.
If you or a loved one is grappling with autosomal dominant polycystic kidney disease (ADPKD), you’ve probably heard the name Tolvaptan comparison floating around. Tolvaptan, sold under the brand Natrise and also known as Tolvaptan, is a big‑ticket medication that promises to slow kidney‑size increase. But it’s pricey, demands regular liver‑function checks, and carries a risk of serious side‑effects. That’s why many patients start looking at “alternatives.” This article breaks down Tolvaptan’s mechanism, its pros and cons, and the most common alternatives-both prescription‑based and lifestyle‑focused-so you can decide what fits your situation best.

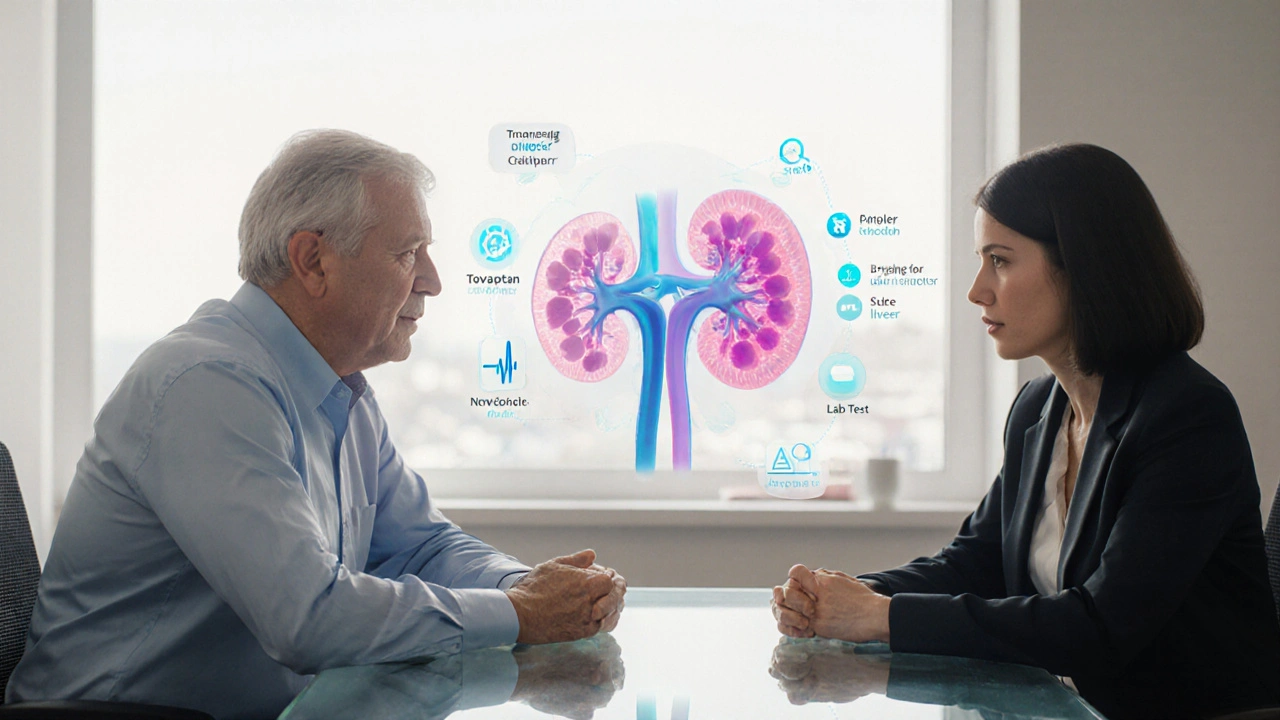





Imagine a world where your kidneys whisper warnings before they swell. Tolvaptan steps in like a daring knight, slowing the relentless march of cysts. Yet the price tag reads like a ransom and liver tests become a weekly ritual. For early‑stage patients with healthy livers the gamble can feel worth the reward. For others the shadows of side effects loom large.
The pharmaceutical giants conceal the true scope of vasopressin antagonists beneath layers of regulatory paperwork. When one examines the clinical trial data patterns emerge that suggest selective disclosure. Tolvaptan's rapid approval appears to serve interests beyond pure science. Patients are entangled in a web of monitoring obligations that feed a larger data economy. The alternative treatments, though modest, escape the spotlight deliberately. One must consider the hidden agenda when choosing therapy.
i read the natrise pamphlet and it felt like a trap for our livers. the side effects are listed but the real risk is hidden in fine print. they say it slows cysts but the cost makes most families give up. i dont trust a drug that needs constant blood work and promises that sound too good to be true.
Hey there, navigating ADPKD treatment can feel like wandering through a maze of medical jargon. Tolvaptan is the only FDA‑approved option that actually puts the brakes on cyst growth, which is huge for early‑stage patients with solid liver function. However, the drug isn’t cheap and the liver‑function monitoring can feel like an extra chore you didn’t sign up for. If you’re comfortable with the surveillance and can handle the potential thirst and liver checks, it can buy you valuable time. On the flip side, blood‑pressure control, low‑salt diet, and staying hydrated are low‑cost strategies that still matter a lot. Talk openly with your nephrologist about your lifestyle, financial situation, and how you feel about regular labs. Together you can craft a plan that balances efficacy with quality of life.
In the grand theater of disease the body plays both protagonist and antagonist. A drug that tempers the villainous cysts does so by meddling with the very hormone that signals water balance. This paradox invites us to ask whether we are correcting a flaw or merely shifting the balance of power within our physiology. Tolvaptan’s promise of slowing kidney enlargement is a double‑edged sword, offering hope while demanding vigilance. As with any intervention, we must weigh the narrative of longevity against the subplot of side‑effects.
Oh great, another pill that needs a spreadsheet of lab results.
They want us to believe Tolvaptan is the silver bullet but the hidden fees are a nightmare. Every dose feels like a secret transaction with the big pharma elite. The liver warnings are just smoke screens to keep us scared enough to stay compliant. Meanwhile the “alternative” diet hacks get shoved under the rug. I swear there’s a whole shadow network deciding who gets the real cure.
Tolvaptan functions as a selective vasopressin V2‑receptor antagonist, thereby attenuating cyclic AMP accumulation in renal tubular epithelial cells. By dampening the intracellular signaling cascade, it reduces epithelial cell proliferation and fluid secretion into cystic compartments. Clinical trials, notably the TEMPO 3:4 study, demonstrated a statistically significant reduction in the rate of total kidney volume increase over a 3‑year horizon. The reported mean annual growth attenuation approximated 49%, translating into a deceleration of eGFR decline. However, the therapeutic benefit is contingent upon stringent inclusion criteria, primarily early‑stage disease with preserved hepatic function. Hepatotoxicity emerged as the principal safety signal, with elevations in alanine aminotransferase and aspartate aminotransferase observed in up to 25% of participants. Consequently, regulatory agencies mandated monthly liver function monitoring for the first 18 months of therapy. Patient adherence is further challenged by aquaretic side‑effects, including polyuria, polydipsia, and nocturia, which can compromise quality of life. The pharmacokinetic profile is characterized by a high degree of inter‑individual variability, necessitating dose titration based on tolerability rather than fixed regimens. Alternative therapeutic avenues, such as the mTOR inhibitors sirolimus and everolimus, have yielded mixed efficacy outcomes and are limited by immunosuppressive adverse events. Somatostatin analogues, including octreotide and lanreotide, have shown modest reductions in cyst volume but lack robust data on long‑term renal function preservation. Lifestyle interventions, notably sodium restriction and rigorous blood pressure control via ACE inhibitors or ARBs, remain cornerstone strategies regardless of pharmacologic therapy. Economic analyses reveal that Tolvaptan’s annual cost can exceed $100,000, posing substantial burden on health‑care systems and patients alike. Insurance coverage heterogeneity further complicates access, prompting many clinicians to prioritize shared decision‑making frameworks. In summary, while Tolvaptan represents a mechanistically targeted option with demonstrable efficacy in selected cohorts, its implementation requires careful risk‑benefit assessment, vigilant hepatic surveillance, and proactive management of aquaretic symptoms.
the natrise story feels like a global puzzle 😊 we see the science but the rollout is shrouded in hidden agendas the liver checks become a ritual that keeps us watching the system
Staying informed and maintaining regular check‑ups can help you navigate those hurdles without feeling overwhelmed.